If physicists can not interact directly with a phenomenon, they often imagine a model for a physical system related to the phenomenon. In this context, a model is a system of physical components, such as electrons and protons in an atom. Once we have identified the physical components, we make predictions about the behavior of the system, based on the interactions between the system components and / or the interaction between the system and the environment outside the system.
For example, consider the behavior of matter. A 1 kg solid gold cube, such as the one to the left of Figure 1.2, is 3.73 cm long. Is this cube nothing more than a golden wall, without empty space? If the cube is cut in half, both pieces retain their chemical identity as solid gold. But what happens if the pieces are cut again and again, indefinitely? Will ever smaller pieces always be made of gold? Such questions go back to the first Greek philosophers. Two of them, Leucippe and his student Democritus, could not accept the idea that such cuts could last forever. They speculated that the process must ultimately end when it produces a particle that can no longer be cut off. Atomos means in Greek "not guilty". From there comes our English word atom.
let us briefly review a number of historical models of the structure of matter. According to the Greek model of the structure of matter, all ordinary matter consists of atoms, as suggested in the lower right corner of the cube of Figure 1.2. Beyond this, no additional structure was specified in the model: the atoms acted as small particles interacting with each other, but the internal structure of the atom was not part of the model.
In 1897, J. J. Thomson identified the electron as a charged and constituent particle of the atom. This led to the first atom model that contained an internal structure. Following the discovery of the nucleus in 1911, a model was developed in which each atom consists of electrons surrounding a central nucleus. A core is shown in Figure 1.2. This model, however, leads to a new question: does the kernel have a structure? In other words, is the nucleus a single particle or a set of particles? The exact composition of the nucleus is not yet fully known, but in the early 1930s a model was developed to help us understand the behavior of the nucleus. Specifically, scientists determined that two basic entities, protons and neutrons, occupied the nucleus. The proton carries a positive electrical charge and a specific chemical element is identified by the number of protons in its nucleus. This number is called the atomic number of the element. For example, the nucleus of a hydrogen atom contains a proton (the number of hydrogen atoms equals 1), the nucleus of a helium atom contains two protons (atomic number 2) and the nucleus a uranium atom contains 92 protons (atomic number 92). In addition to the atomic number, there is a second number characterizing the number of atomic mass, defined as the number of protons plus neutrons in a nucleus. The atomic number of an element never varies (that is, the number of protons does not vary), but the mass number may vary (that is, the number of neutrons varies)
The existence of neutrons was conclusively verified in 1932. A neutron has no charge and its mass is about equal to that of a proton. One of its main goals is to act as a "glue" that keeps the core together. If the neutrons were not present in the nucleus, the repulsive force between the positively charged particles would cause the separation of the nucleus. But is this where the process of decomposition stops? Protons, neutrons and a host of other exotic particles are now known to be made up of six different varieties of particles called quarks, which have the names of high, low, strange, charmed, low and high. The high quarks, charmed and superior have +2/3 charges of the proton, while low, strange, and lower quarks have -1/3 charges of the proton. The proton consists of two high quarks and one low quark, as shown at the top of Figure 1.2. You can easily show that this structure predicts the correct charge of the proton. Similarly, the neutron consists of two downstream quarks and one upstream quark, giving a net charge of zero.
This model building process is a process you should develop when you study physics. You will be faced with many mathematical problems to solve in this study. One of the most important techniques is to develop a model for the problem, to identify a system of physical components, and to predict system behavior based on interactions between system components and/or interaction between components system and its environment.
This model building process is a process you should develop when you study physics. You will be faced with many mathematical problems to solve in this study. One of the most important techniques is to develop a model for the problem, to identify a system of physical components, and to predict system behavior based on interactions between system components and/or interaction between components system and its environment.
Density and Atomic Mass
In section 1.1, we explored three basic quantities in mechanics. Let's look at an example of a derived quantity-density. The density & (Greek letter rho) of any substance is defined as its mass per unit volume
For example, aluminium has a density of 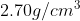 , and lead has a density of
, and lead has a density of 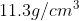 . Therefore, a piece of aluminium of volume 10.0 cm^3 has a mass 0f 27.0 g, Therefore, a piece of aluminum with a volume of 10.0 cm 3 has a mass of 27.0 g, while an equivalent volume of lead has a mass of 113 g. A list of densities for different substances is given in the table
. Therefore, a piece of aluminium of volume 10.0 cm^3 has a mass 0f 27.0 g, Therefore, a piece of aluminum with a volume of 10.0 cm 3 has a mass of 27.0 g, while an equivalent volume of lead has a mass of 113 g. A list of densities for different substances is given in the table
The number of protons and neutrons in the nucleus of the atom of an element is related to the atomic mass of the element, defined as the mass of a single atom of the element measured in units of atomic mass (u ) where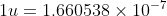 kg.
kg.
The number of protons and neutrons in the nucleus of the atom of an element is related to the atomic mass of the element, defined as the mass of a single atom of the element measured in units of atomic mass (u ) where
The atomic mass of lead is 207 u and that of aluminum is 27.0 u. However, the ratio of atomic masses, 207 u/27.0 u = 7.67, does not correspond to the ratio of densities,
Reference
Serway R.A and Jewett J.W. 2004. Physics For Scientists and Engineers 6th Edition. ISBN: 0534408427. Thomson Brooks/Cole.


